Johnson And Johnson Vaccine Kids - Man's skin 'peeled off' in rare reaction to Johnson ... / Johnson & johnson have now start enrolling children in its vaccine trial that is set to begin in houston in a few weeks.
Johnson And Johnson Vaccine Kids - Man's skin 'peeled off' in rare reaction to Johnson ... / Johnson & johnson have now start enrolling children in its vaccine trial that is set to begin in houston in a few weeks.. Surgeon general vivek murthy about why covid vaccinations with the johnson & johnson vaccine have been put on hold, the nature of the blood clot concerns, and what the pause means for people who have. Johnson & johnson plans to test its coronavirus vaccine in infants and even in newborns, as well as in pregnant women and in people who have compromised immune systems. Johnson & johnson have now start enrolling children in its vaccine trial that is set to begin in houston in a few weeks. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. It confirmed that one patient.
The fda said it was recommending the temporary pause out of an abundance of caution. Here, we give a rundown of basic facts about the vaccine and an overview of how it works. Chandan khanna/afp via getty images. Johnson & johnson plans to test its coronavirus vaccine in infants and even in newborns, as well as in pregnant women and in people who have compromised immune systems. The johnson & johnson vaccine also has a unique benefit:
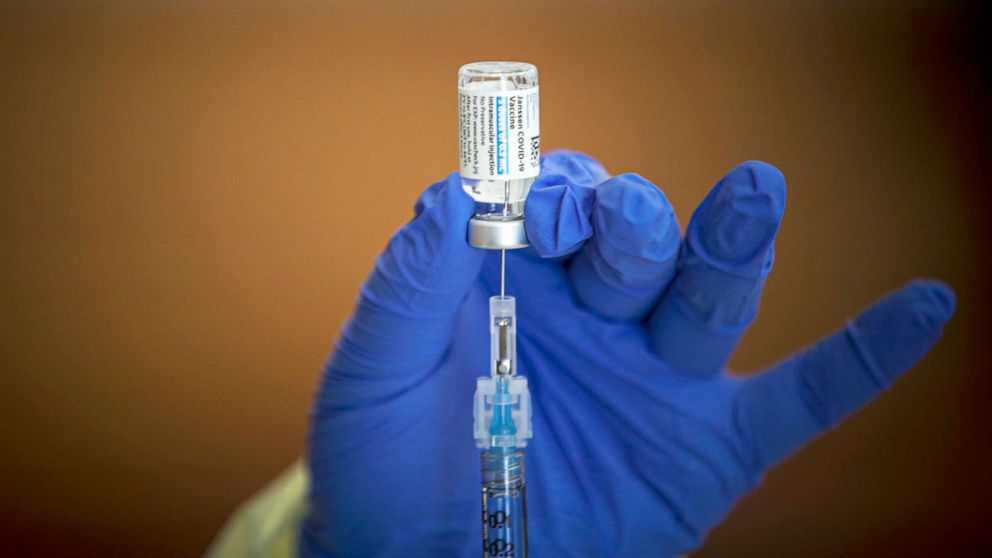
You only need one dose of it.
Janssen pharmaceuticals companies of johnson & johnson. It follows similar cases after doses of the astrazeneca vaccine, which prompted curbs to its use. The johnson & johnson vaccine also has a unique benefit: But much of the debate over the next weeks is likely to be about how to best use a vaccine with a different efficacy profile and. Johnson & johnson has paused its eu rollout, which started this week. Ofer levy, director of the precision vaccines. Johnson & johnson confirmed in a statement released tuesday that the vaccine formula itself includes no fetal tissue. It confirmed that one patient. The cdc and fda called for a temporary but immediate halt to the use of j&j's covid vaccine while they investigate at least six cases of potentially dangerous blood clots in people who received the vaccine. Here, we give a rundown of basic facts about the vaccine and an overview of how it works. Johnson & johnson have now start enrolling children in its vaccine trial that is set to begin in houston in a few weeks. Surgeon general vivek murthy about why covid vaccinations with the johnson & johnson vaccine have been put on hold, the nature of the blood clot concerns, and what the pause means for people who have. Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher.
The cdc and fda called for a temporary but immediate halt to the use of j&j's covid vaccine while they investigate at least six cases of potentially dangerous blood clots in people who received the vaccine. But much of the debate over the next weeks is likely to be about how to best use a vaccine with a different efficacy profile and. The particular cells that are involved in the johnson & johnson. A vaccine is unlikely to be ready before 2021. Johnson & johnson confirmed in a statement released tuesday that the vaccine formula itself includes no fetal tissue.

Janssen pharmaceuticals companies of johnson & johnson.
Chandan khanna/afp via getty images. The bold plan for expanded clinical trials met with the approval of dr. It follows similar cases after doses of the astrazeneca vaccine, which prompted curbs to its use. The particular cells that are involved in the johnson & johnson. Johnson & johnson has paused its eu rollout, which started this week. It confirmed that one patient. The latest casualty is johnson & johnson, which halted its trial due to an unexplained illness in one of its participants. But much of the debate over the next weeks is likely to be about how to best use a vaccine with a different efficacy profile and. The fda said it was recommending the temporary pause out of an abundance of caution. Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher. Johnson & johnson confirmed in a statement released tuesday that the vaccine formula itself includes no fetal tissue. Ofer levy, director of the precision vaccines. The j&j/janssen vaccine is recommended for people aged 18 years and older.
Surgeon general vivek murthy about why covid vaccinations with the johnson & johnson vaccine have been put on hold, the nature of the blood clot concerns, and what the pause means for people who have. It confirmed that one patient. Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher. Johnson & johnson have now start enrolling children in its vaccine trial that is set to begin in houston in a few weeks. The j&j/janssen vaccine is recommended for people aged 18 years and older.
The johnson & johnson vaccine also has a unique benefit:
Here's a closer look at its technology, efficacy. Johnson & johnson has paused its eu rollout, which started this week. The johnson & johnson vaccine also has a unique benefit: Johnson and johnson will start their tests on kids between 12 and 18, and then will go younger as the vaccine trials go on. Johnson & johnson plans to test its coronavirus vaccine in infants and even in newborns, as well as in pregnant women and in people who have compromised immune systems. Johnson & johnson has a separate ongoing trial of two doses of the vaccine, because studies have demonstrated that a second dose can push the immune response even higher. Here, we give a rundown of basic facts about the vaccine and an overview of how it works. The bold plan for expanded clinical trials met with the approval of dr. Johnson & johnson have now start enrolling children in its vaccine trial that is set to begin in houston in a few weeks. It confirmed that one patient. The cdc and fda called for a temporary but immediate halt to the use of j&j's covid vaccine while they investigate at least six cases of potentially dangerous blood clots in people who received the vaccine. The particular cells that are involved in the johnson & johnson. Rachel maddow, who received the johnson & johnson vaccine a week ago, talks with u.s.
Komentar
Posting Komentar